Chronic rhinosinusitis is one of the most prevalent chronic disorders in the US, affecting 10-15% of the population and all age groups. A significant number of these patients suffer from the growth of fleshy swellings or polyps in the lining of the nose and paranasal sinuses. For some, surgery and medical treatments do not offer sufficient benefits.

Washington University rhinologists John Schneider, MD, Cristine Klatt-Cromwell, MD, and Nyssa Farrell, MD, are seeing benefits of biologic therapy for their chronic patients when other approaches have failed.
Biologic therapy or biologics refer to any type of medical therapy that is derived from living organisms such as humans, animals or microorganisms. Monoclonal antibodies are one form of biologics that are increasingly used to treat autoimmune disorders and cancer. These antibodies are used to activate or block cell receptors in order to control cell activity. By doing so they can be used to activate or suppress an overactive immune system.
According to Dr. Farrell, biologics are reserved for those patients that have been unable to achieve sufficient control through surgery or topical medications like corticosteroids.
“It can be especially helpful for patients that exhibit associated symptoms like asthma, inflammation, or aspirin-exacerbated respiratory disease,” she said. “Dupilumab is the biologic currently approved by the FDA for chronic rhinosinusitis with nasal polyps.”
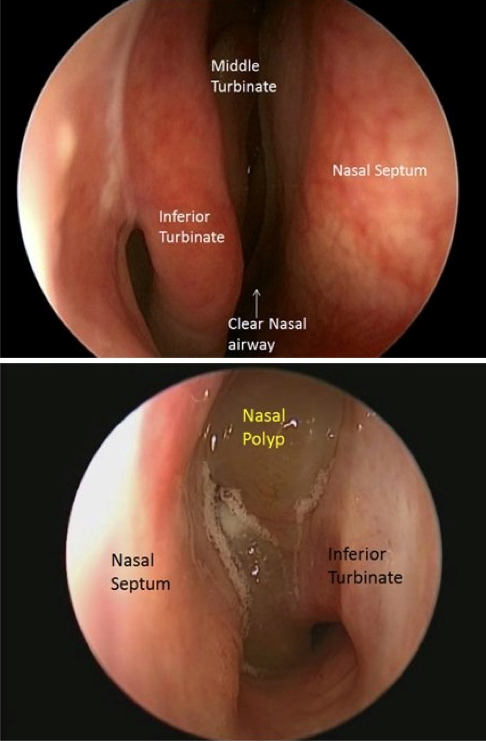
The European Forum for Research and Education in Allergy and Airway Diseases is an international non-profit forum of doctors, hospitals, researchers and patients. They recently released a statement on the role of biologics for these patients. They concluded that biologic therapy is indicated in patients with bilateral nasal polyps who had previously undergone sinus surgery and met three of the following criteria:
- evidence of Type 2 inflammation
- need for systemic corticosteroids in the past two years
- significant quality-of-life impairment
- loss of smell
- sinusitis-related asthma
In patients who have never had sinus surgery, at least four of the above criteria should be met in order to be eligible for biological treatment.
Dupilumab inhibits two cell signaling molecules or interleukins, called Il-4 and Il-13, which contribute to inflammatory processes. By blocking these pathways, biologic therapy can calm over-reactive immune responses without necessarily suppressing the immune system. Other biologicals are in clinical trial testing that work by blocking other triggers of inflammation.
How effective can biologic therapy be for these patients? According to Dr. Farrell, this is a hard question to answer. “We are still working to identify the best patients for biologic therapy, she said. Prior studies have positive, though variable results, but overall I would say that biologics are very promising and provide a great option for these patients.”
