Peng Lab

Our Mission
Our mission is to understand the role of the immune system in the pathogenesis of cancer and age-related neurological disorders, and then to develop effective strategies for the manipulation of immune cell fate and function to provide disease prevention and immunotherapy.
Research Projects
1. Evaluating the role of different tumor-infiltrating T cell subsets in the suppressive tumor microenvironment for tumor immunity
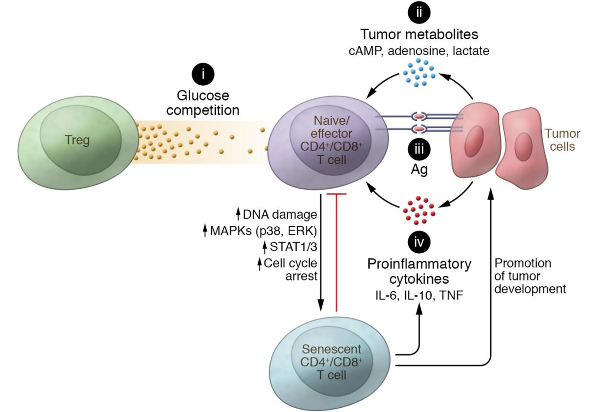
Clinical data collectively suggest that cancer-specific immune responses can be induced, but such responses are too weak and transient to kill tumor cells. One of the key determinants for the therapeutic efficacy and immune responses is the functional state of the transferred/preexisting T cells in the suppressive tumor microenvironment. Our studies directly addressed the important issues of identifying the roles of tumor-infiltrating T cells in the maintenance of immunological tolerance and tumor-suppressive microenvironment in different types of tumors, including head & neck, lung, colon, breast cancers, and melanoma, and further exploring the related molecular mechanisms. We have demonstrated that both CD4+ and CD8+ tumor-specific Treg cells, as well as gd Treg cells, are present at tumor sites that strongly suppress immune responses. To explore the molecular mechanisms responsible for T cell dysfunction in the tumor microenvironment, we have more recently discovered that the induction of T cell senescence is a novel suppressive mechanism mediated by naturally occurring and tumor-derived Treg cells, as well as multiple types of tumor cells. Importantly, these senescent T cells are functional suppressive, and molecularly distinct from anergic and exhausted T cells. Our goals are: a) to explore the roles and molecular interactions of those regulatory immune cell components in the tumor microenvironment; b) to develop novel strategies to block immune suppression mediated by regulatory immune cells; and c) to prevent the generation of and promote functional rejuvenation of senescent tumor-specific effector T cells for tumor immunotherapy.
2. Metabolic reprogramming of cell fate and function of tumor-specific T cells for tumor immunotherapy
It is now clear that metabolic reprogramming is a hallmark of cancers, and the metabolism of tumor cells differs from healthy or normal cells because of the acquisition and maintenance of their malignant properties. Cellular energy metabolism also can direct T cell survival, proliferation, and their specific functions. Furthermore, unique T cell subsets have specialized metabolic profiles. Meanwhile, tumor-infiltrating T cells are also encountering inhibition from tumor-derived metabolic waste products and low oxygen. We have recently demonstrated that tumor-derived Treg cells can mediate the acceleration of glucose consumption and trigger cell senescence and DNA damage in responder T cells during their cross-talk. We further identified that overaction of lipid metabolism in responder effector T cells induced by Treg and tumor cells leads to T cell senescence. Therefore, comprehensively exploring the metabolic profiles of T cells and the causative molecular interactions within the suppressive tumor microenvironment will facilitate the development of novel strategies for cancer therapy via metabolic reprogramming of cell fate and functions. In addition, understanding how tumor-derived metabolites influence T cell differentiation and function seeks to provide novel therapeutic approaches.
3. Identifying novel checkpoint molecules for the development of effective strategies for tumor immunotherapy
Immunotherapies have resulted in promising results in cancer patients, but efficacy is highly variable among different tumor types. Current checkpoint blockade therapy using antibodies targeting PD1/PDL1 or/and CTLA4 has exhibited limited success rates from 15% to 35%, further suggesting that there are other mechanisms and/or checkpoint signaling pathways involved in T cell dysfunction mediated by malignant tumors. We will use state-of-the-art strategies, such as scRNA-seq and multiplex spatial profiling to explore unique immune signatures of immunotherapy response and non-response, as well as to identify alternative checkpoint molecules for the development of effective strategies for tumor immunotherapy in different types of cancers, including head & neck, lung, colon, breast cancers, and melanoma.
4. Dissecting the role of senescent T cells in Alzheimer’s disease
Alzheimer’s disease (AD) is the most common form of both age-associated dementia and neurodegenerative disorder representing a major public health priority. An improved understanding of the molecular and cellular processes responsible for the pathogenesis of AD is urgently needed, which could lead to the development of novel and effective diagnostic and therapeutic strategies. Studies from our group and others suggest that a dysfunctional and aging immune system may be a primary factor for the development of AD. Increased senescent CD4+ and CD8+ T cells have been shown in peripheral blood of AD patients. We further discovered that accumulated senescent T cells exist in aged normal mice and in aged spontaneous senescence-accelerated mouse P8 (SAMP8) and 5XFAD AD mice. Importantly, we found that senescent T cells can promote the aggregation of amyloid precursor protein (APP), amyloid beta (Aβ), and Tau proteins in human neuronal cells. However, the causative role of senescent T cell populations in the pathogenesis of AD is unknown. Our long-term goal is to explore the role of immune cell components in the pathogenies of AD and then develop strategies to modify their molecular interactions to improve AD prevention and treatment.
Research Support




Team Members

Professor of Otolaryngology
pengg@wustl.edu

Staff Scientist
white.jason@wustl.edu

Postdoctoral Researcher
yuanqin@wustl.edu

Assistant Professor
lxia@wustl.edu

Postdoctoral Researcher

Lab Manager
zhaolei@wustl.edu

Research Specialist
Undergraduate student

Undergraduate student

Undergraduate student
Select Publications
- Zhao Y, Liu X, Si F, Huang L, GaoA, Lin W, Hoft DF, Shao Q, Peng G. Citrate promotes excessive lipid biosynthesis and senescence in tumor cells for tumor therapy. Advanced Science. 2022, Jan; 9(1): e2101553. doi: 10.1002/advs.202101553.
- Liu X, Hoft DF, Peng G. Tumor microenvironment metabolites directing T cell differentiation and function. Trends Immunol. 2022 Feb; 43(2): 132-147. doi: 10.1016/j.it.2021.12.004.
- Liu X, Si F, Bagley D, Ma F, Zhang Y, Tao Y, Shaw E, Peng G. Blockades of effector T cell senescence and exhaustion synergistically enhance anti-tumor immunity and immunotherapy. J Immunother Cancer. 2022 Oct; 10(10): e005020. doi: 10.1136/jitc-2022-005020.
- Liu X, Li L, Si F, Huang L, Zhao Y, Zhang C, Hoft DF, Peng G. NK and NKT cells have distinct properties and functions in cancer. Oncogene.2021 Jul; 40(27): 4521-4537. doi: 10.1038/s41388-021-01880-9.
- Gao A, Liu X, Lin W, WangJ, Wang S, Si F, HuangL, Zhao Y, Sun Y, Peng G.Tumor-derived ILT4 induces T cell senescence and suppresses tumor immunity. J Immunother Cancer. 2021 Mar; 9(3): e001536. doi: 10.1136/jitc-2020-001536.
- Liu X, Hartman CL, Li L, Albert CJ, Si F, Gao A, Huang L, Zhao Y, Lin W, Hsueh EC, Shen L, Shao Q, Hoft DF, Ford DA, Peng G. Reprogramming lipid metabolism prevents effector T cell senescence and enhances tumor immunotherapy. Sci. Transl. Med. 2021 Mar 31; 13(587): eaaz6314. doi: 10.1126/scitranslmed.aaz6314.
- Liu X, Hoft DF, Peng G. Senescent T cells within suppressive tumor microenvironments: Emerging target for tumor immunotherapy. Journal of Clinical Investigation. 2020 Mar 2; 130(3):1073-1083. doi: 10.1172/JCI133679.
- Li L, Liu X, Sanders KL, EdwardsJ, YeJ, Si F, Gao A, Huang L, Hsueh EC, Ford DA, Hoft DF, Peng G. TLR8-mediated metabolic control of human Treg function: A mechanistic target for cancer immunotherapy. Cell Metabolism. 2019 Jan 8; 29(1): 103-123. doi: 10.1016/j.cmet.2018.09.020.
- Ma C, Wang F, Han B, Zhong X, Si F, Ye J, Hsueh EC, Robbins L, Kiefer SM, Zhang Y, Hunborg P, Varvares MA, Rauchman M, Peng G. SALL1 functions as a tumor suppressor in breast cancer by regulating cancer cell senescence and metastasis through the NuRD complex. Molecular Cancer. 2018 Apr 6; 17(1): 78. doi: 10.1186/s12943-018-0824-y.
- Liu X, Mo W, Ye J, Li L, Zhang Y, Hsueh EC, Hoft DF, Peng G. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nature communications. 2018 Jan 16; 9(1): 249. doi: 10.1038/s41467-017-02689-5.
- Ye J, Ma C, Hsueh EC, Dou J, Mo W, Liu S, Han B, Huang Y, Zhang Y, Varvares MA, Hoft DF, Peng G. TLR8 signaling enhances tumor immunity by preventing tumor-induced T-cell senescence. EMBO Mol Med. 2014 Oct; 6(10): 1294-311. doi: 10.15252/emmm.201403918.
- Ye J, Huang X, Hsueh EC, Zhang Q, Ma C, Zhang Y, Varvares MA, Hoft DF, Peng G. Human regulatory T cells induce T-lymphocyte senescence.Blood. 2012 Sep 6; 120(10): 2021-31. doi: 10.1182/blood-2012-03-416040.
- Peng G, Guo Z, Kiniwa Y , Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005 Aug 26; 309(5739):1380-4. doi: 10.1126/science.1113401.
- Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH,Wang RF. Tumor-Infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007 Aug; 27(2): 334-48. doi: 10.1016/j.immuni.2007.05.020.
- Wang HY, Lee DA, Peng G, Guo Z,Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004 Jan; 20(1): 107-18. doi: 10.1016/s1074-7613(03)00359-5.
Contact Us
Physical Location: We are located in Rooms 1902/1905 in the South Medical Building on the medical school campus of Washington University.
4577 McKinley Ave.
St. Louis, MO 63110
Mail Address:
Guangyong Peng, MD, PhD
Department of Otolaryngology
Washington University School of Medicine in St. Louis
MSC 8115-003-1902/1905
St. Louis, MO 63110, USA
Lab Telephone: 314-273-3147
Email: pengg@wustl.edu
Lab Opportunities
We are always looking for highly motivated and passionate graduate students, post-doctoral fellows, staff scientists, and technicians with strong research experience and excellent educational backgrounds who want to join our team!
Postdoctoral Researchers
The laboratory of Dr. Guangyong Peng has an immediate opening for two Postdoctoral Associate positions to play an active role in our ongoing and NIH-funded studies of tumor immunology, neuroimmunology, and T-cell biology.
Highly motivated and passionate researchers with a M.D. or Ph.D. degree and rigorous training in immunology, cancer biology, neurology, or related areas are preferred for the positions. Furthermore, the candidates should have experience with immunology and be comfortable working with mice and/or patient samples. In addition, candidates with research experience with bioinformatics and next-generation sequencing are preferred but not required. These positions are full-time appointments with all benefits provided by Washington University in St. Louis. Salary is according to the NIH standard level. Funding is for at least 3 years with additional years possible. Washington University in St. Louis is an Equal Opportunity/Affirmative Action Employer.
Candidates must apply with a cover letter and current curriculum vitae to https://jobs.wustl.edu. Meanwhile, applicants need to submit the application and three letters of recommendation by email to: Guangyong Peng, MD, PhD, Email: pengg@wustl.edu. Review of applications begins immediately and continues until the positions are filled.
Graduate students
We are always looking for passionate, creative, and motivated students. Individuals with a strong interest in tumor immunology, cancer biology, and neuroimmunology are welcome to join the Peng lab!
Graduate students from the DBBS programs in Immunology, Cancer Biology, and Neurosciences at Washington University are welcome to rotate and join our lab.