
Title: “Cochlear Implantation in Children with Asymmetric Hearing Loss or Single-Sided Deafness Clinical Trial”
What it will study: This clinical trial is designed as a longitudinal, prospective, two-phase, multi-center clinical trial to address deficits in children who have either asymmetric hearing loss or single-sided deafness. The clinical trial will assess cochlear implantation as a treatment using measures of speech recognition, localization and quality of life at five US sites. Results of the study will inform the development of clinical protocols and aid in clinical decisions concerning treatment and rehabilitation for two substantially underserved pediatric populations.
Funding Agency: NIH/NIDCD (National Institute on Deafness and Other Communication Disorders)
Amount: $866,945
Co-PI/Collaborator: Craig Buchman, MD; Michael Strube, PhD; Ruth Reeder, MS, Laura Holden, AuD, Noel Dwyer, AuD, Tim Holden, BSE, Lisa Davidson, PhD, Judith Lieu, MD, Keiko Hirose, MD.
Subsites PIs: Laurie Eisenberg, PhD, University of Southern California; John Germiller, MD, Children’s Hospital of Pennsylvania; Jace Wolfe, PhD, Hearts for Hearing, Oklahoma City, OK; Kristin Gravel, AuD, University of Minnesota; Jamie Cadieux, AuD, St. Louis Children’s Hospital.

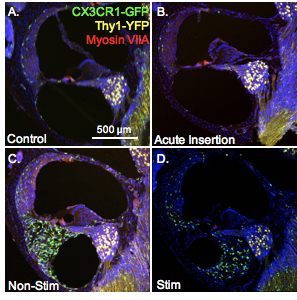
Title: “Role of Macrophages on Tissue Remodeling Following Cochlear Implantation”
What it will study: Functional and structural changes of the cochlea after cochlear implantation in mice, specifically with the goal of better understanding how the inflammatory response contributes to changes in the inner ear after surgery, after chronic implantation, and after electrical stimulation of the inner ear.
Funding Agency: National Institutes of Health (NIDCD)
Amount: $434,565
Collaborator/Co-PI: Marlan R. Hansen, MD at the University of Iowa

Title: “Role of the Innate Immune System in The Survival of Auditory Neurons”
What it will study: Studies of aging in humans have suggested a link between inner ear disorders and the development of Alzheimer’s disease. We have shown that amyloid precursor protein (APP, which is implicated in neurodegeneration in Alzheimer’s disease) is highly expressed in the sensory organs and neurons of the inner ear. This project will determine whether mutations in APP lead to increased degeneration and inflammation in the inner ear and auditory brainstem.
Funding Agency: National Institutes of Health (NIDCD)
Amount: $404,322
Collaborator/Co-PI: Co-PI Edwin Rubel, PhD, University of Washington

Title: “Development of an in vivo model system for the study of sensory afferent regeneration”
What it will study: Uses the zebrafish model to identify strategies for stimulating in vivo replacement of lost cochlear nerves in humans.
Funding Agency: McDonnell Center for Cellular and Molecular Neurobiology Small Grants Funding
Amount: $36,000
Collaborator/co-PI: Mark Warchol PhD
Lavinia Sheets, PhD
Title: “Prevention of sensory pathology following cisplatin chemotherapy”
What it will study: Uses the zebrafish model to investigate pharmacological therapies for minimizing cisplatin-induced damage to both hair cells and afferent nerves of the inner ear.
Funding Agency: Children’s Discovery Institute of Washington University at St. Louis Children’s Hospital
Amount: $121,000 for year 3
Collaborator/Co PI: Mark Warchol, PhD

Title: “Invasion and metastasis in head and neck cancer”
What it will study: Dr. Puram’s partial EMT gene signature and its role in nodal metastasis as well as new studies exploring neoantigens and head and neck tumor immunology.
Funding Agency: Doris Duke Fund to Retain Clinical Scientists
Amount: $50,000
Sidharth Puram, MD, PhD
Title: “A partial epithelial-to-mesenchymal signature for predicting nodal metastasis”
What it will study: Dr. Puram will attempt to identify the most promising markers of the p-EMT signature and whether this predicts outcomes in patients through additional studies in new patients, and thereby determine if this gene signature can be translated to the clinic.
Funding Agency: AAOHNS Bobby R. Alford Endowed Research Grant (The American Academy of Otolaryngology-Head & Neck Surgery)
Amount: $30,000

Title: “Targeting calcium-permeable AMPA receptors for inner ear therapy”
Study: First-ever drug for the prevention of hearing loss and/or tinnitus
Funding Agency: Washington University’s Skandalaris LEAP fund
Amount: $40,000
Co PIs: Steven Green, PhD, professor of Biological Sciences and Otolaryngology at the University of Iowa; Roland Dulle, PhD, chemistry director, Center for Drug Discovery, Washington University School of Medicine
