Rutherford Lab
Sensory encoding and excitotoxicity
Principal Investigator:
Mark A. Rutherford, PhD, Assistant Professor, Otolaryngology-Head & Neck Surgery
Overview
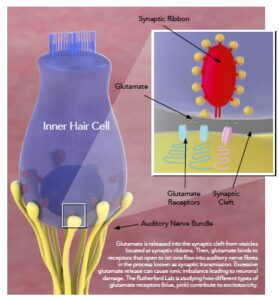 The Rutherford Lab is focused on mechanisms of glutamate signaling in the cochlea. With basic and translational science approaches, we study how sound is transduced by the inner ear into action potentials in the auditory nerve through mechanisms of synaptic transmission. While the neurotransmitter glutamate is necessary for hearing, it is also responsible for synaptopathy. We are developing novel compounds that allow for hearing function to be maintained while preventing the damaging effects of glutamate in noisy environments. To make a gift to support the work of the lab, please contact Dr. Rutherford directly, or see the school of medicine page on giving for more information.
The Rutherford Lab is focused on mechanisms of glutamate signaling in the cochlea. With basic and translational science approaches, we study how sound is transduced by the inner ear into action potentials in the auditory nerve through mechanisms of synaptic transmission. While the neurotransmitter glutamate is necessary for hearing, it is also responsible for synaptopathy. We are developing novel compounds that allow for hearing function to be maintained while preventing the damaging effects of glutamate in noisy environments. To make a gift to support the work of the lab, please contact Dr. Rutherford directly, or see the school of medicine page on giving for more information.
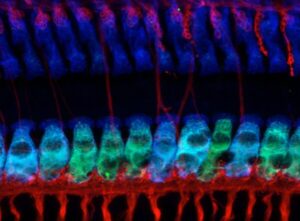 Excitotoxicity. During overexposure to sound, excessive release of glutamate from the sensory receptors (inner hair cells) drives an excitotoxic response that causes synaptic disintegration and auditory nerve degeneration. Our experiments are elucidating the mechanisms of glutamatergic excitotoxicity in the cochlea.
Excitotoxicity. During overexposure to sound, excessive release of glutamate from the sensory receptors (inner hair cells) drives an excitotoxic response that causes synaptic disintegration and auditory nerve degeneration. Our experiments are elucidating the mechanisms of glutamatergic excitotoxicity in the cochlea.
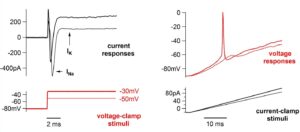
Sensory Encoding. Auditory nerve fibers of the cochlea differ greatly in sound-response properties. In addition, they differ in excitotoxic vulnerability. Our overall goal is to know what makes them different, considering the combined effects of presynaptic, postsynaptic, and spike generator heterogeneities.
Relevance
Hearing loss reduces quality of life and is associated with acceleration of cognitive decline. Approximately 17% of Americans have some degree of hearing loss, predominantly due to noise exposure, and 47% of adults 75 years or older have a hearing loss due to ‘normal’ lifetime exposure. These staggering statistics highlight the need for a better understanding of the cellular processes that underlie use-dependent damage to the cells of the inner ear.
Goals
Our sense of hearing is extremely sensitive to vibrations at the nanometer scale, allowing for detection of very low-level sounds. At the same time, our ears must deal with very loud sounds, spanning an intensity range of 12 orders of magnitude. The world is noisy and getting noisier, but humans did not evolve in such a noisy environment. Cochlear hair cells and the auditory nerve fibers they excite have little to no capacity for spontaneous regeneration, so damage to our sense of hearing is often permanent. Therefore, our goal is to prevent hearing loss by protecting the cochlea from excitotoxicity. We are learning how to avoid damage to the specialized sensory synapses of the cochlea, while maintaining hearing function. In the lab we apply state-of-the-art research tools to define the normal mechanisms of cochlear function in order to explain and prevent disease.
Research projects
- Molecular anatomy of glutamate receptors at mammalian hair cell synapses using advanced microscopy in collaboration with the Wash. U. Center for Cellular Imaging.
- Glutamate receptor pharmacology using Ca2+ imaging and patch-clamp electrophysiology.
- Drug discovery / lead optimization in collaboration with the Wash. U. Center for Drug Discovery.
- Mechanisms of synaptic transmission and action potential generation that underlie sensory encoding in auditory hair cells and neurons.
- The relationship between hearing loss and Alzheimer’s disease in collaboration with Wash. U. Dept. of Neurology.
- Development of better cochlear prosthetics through biophysical modeling of the auditory nerve.
Collaborations within the excellent research environment at Washington University support these aims, as well as collaborations with several domestic and foreign universities.
Lab Team
- Mark A. Rutherford, PhD
- Amit Walia, MD, PhD
- Maolei Xiao, PhD
- Shelby Payne, BS
Undergrads
- We have enjoyed working with many Wash. U. undergraduate students over the years, including included Guhan Iyer, Jason Carlquist, Sonali Guttani, Honey Patel, Natalie Skigen, Matt Nester, Kya Vaughn, Devon Chen, Sofia Kling, Heather Chung, and Atri Battacharyya.
Audiology Graduate Students
- Allison Schwed, AuD
- Bethany Davis, AuD
Graduate Alumni
- Luis E. Boero, PhD
Postdoctoral Alumni
- Kyunghee X. Kim, PhD
- Tzu-Lun Ohn, PhD
- Jay Gantz, MD, PhD
- Babak V-Ghaffari, PhD
Current Funding
2016-2021: NIDCD R01. Excitability and Excitotoxicity in Type I Cochlear Afferents: Synaptic Structure and Function.
2020: NIA R01 Supplement. Alzheimer’s Disease Supplement for Grants Not Focused on Alzheimer’s Disease: Excitation and Excitotoxicity in Type I Cochlear Afferents: Synaptic Structure and Function
Select Publications
- Hu, N., Rutherford, M.A. and Green, S.H. Protection of cochlear synapses from noise-induced excitotoxic trauma by blockade of Ca2+-permeable AMPA receptors. PNAS 117 (7): 3828 (2020). PMID: 32015128.
- Lingle, C.J., Martinez-Espinosa, P.L., Yang-Hood, A., Boero, L., Payne, S., Persic D., V-Gharffari, B., Xiao, M., Zhou, Y., Xia, X.M., Pyott, S.J., Rutherford, M.A. LRRC52 regulates BK channel function and localization in mouse cochlear inner hair cells. PNAS 116 (37): 18397 (2019). PMID: 31451634.
- Kyunghee X. Kim, Shelby Payne, Aizhen Yang-Hood, Song-Zhe Li, Bethany Davis, Jason Carlquist, Babak V-Ghaffari, Jay A. Gantz, Dorina Kallogjeri, James A. J. Fitzpatrick, Kevin K. Ohlemiller, Keiko Hirose, and Mark A. Rutherford. Vesicular Glutamatergic Transmission in Noise-induced Loss and Repair of Cochlear Ribbon Synapses. J Neurosci 2019 PMID: 30926748
- Choongheon Lee, John Guinan, Mark Rutherford, Wafaa Kaf, Kaitlyn Kennedy, Craig Buchman, Alec Salt, and Jeffery Lichtenhan. Cochlear compound action potentials from high-level tone bursts originate from wide cochlear regions that are offset toward the most sensitive cochlear region. JNeurophysiol 121(3): 1018 (2019). PMID: 30673362
- Becker, L., Schnee M.E., Niwa, M., Sun W., Maxeiner S., Talaei S., Kachar B., Rutherford M.A., and Ricci, A.J. The presynaptic ribbon maintains vesicle populations at the hair cell afferent fiber synapse. eLife 7 (2018): e30241. PMID: 29328021
- Sebe, J.Y., Cho, S., Sheets, L., Rutherford, M.A., von Gersdorff, H., and Raible, D.W. Ca2+-Permeable AMPARs Mediate Glutamatergic Transmission and Excitotoxic Damage at the Hair Cell Ribbon Synapse. The Journal of Neuroscience 37.25 (2017): 6162-6175. PMID: 28539424
- Hirose, K., Rutherford, M.A., and Warchol, M.E. Two cell populations participate in clearance of damaged hair cells from the sensory epithelia of the inner ear. Hearing Research (2017). PMID: 28526177
- Ohn, T.L., Rutherford, M.A., Jing, Z., Jung, S., Duque-Afonso, C.J., Hoch, G., Picher, M.M., Scharinger, A., Strenzke, N., and Moser, T. Hair Cells Use Active Zones with Different Voltage Dependence of Ca2+ Influx to Decompose Sounds into Complementary Neural Codes. PNAS 113(32): E4716 (2016) PMID: 2746210.
- Kim, Kyunghee X., and Mark A. Rutherford. “Maturation of NaV and KV Channel Topographies in the Auditory Nerve Spike Initiator before and after Developmental Onset of Hearing Function.” The Journal of Neuroscience 36.7 (2016): 2111-2118.
- Rutherford, M.A. and Moser, T. (2016) “The Ribbon Synapse Between Type I Spiral Ganglion Neurons and Inner Hair Cells” In: The Primary Auditory Neurons of the Mammalian Cochlea. Springer Handbook of Auditory Research. Eds. A. Dabdoub and B. Fritzsch. DOI 10.1007/978-1-4939-3031-9_5
- Rutherford, M.A. “Resolving the structure of inner ear ribbon synapses with STED microscopy.” Synapse 69.5 (2015): 242-255.
- Wong, A.B., Rutherford, M.A., Gabrielaitis, M., Pangršič, T., Göttfert, F., Frank, T., Michanski, F., Hell, S., Wolf, F., Wichman, C., Moser, T. Developmental Refinement of Hair Cell Synapses Tightens the Coupling of Ca2+ Influx to Exocytosis. EMBO J 33(3):247 (2014).
- Wong, A.B., Jing, Z., Rutherford, M.A., Frank, T., Strenzke, N., Moser, T. Concurrent Maturation of Inner Hair Cell Synaptic Ca2+ Influx and Auditory Nerve Spontaneous Activity around Hearing Onset in Mice. J Neurosci 33(26):10661 (2013).
- Jing, Z., Rutherford, M.A., Takago, H., Frank, T., Fejtova, A., Khimich, D., Moser, T., Strenzke, N. Disruption of the Presynaptic Cytomatrix Protein Bassoon Degrades Ribbon Anchorage, Multi-quantal Release, and Sound Encoding at the Hair Cell Afferent Synapse. J Neurosci 33(10):4456 (2013).
- von Ameln S, Wang G, Boulouiz R, Rutherford MA, Smith GM, Li Y, Pogoda HM, Nürnberg G, Volk AE, Stiller B, Hong JS, Goodyear RJ, Nürnberg P, Richardson GP, Hammerschmidt M, Moser T, Wollnik B, Koehler CM, Teitell MA, Barakat A, Kubisch C (2012) A Mutation in PNPT1, Encoding Mitochondrial-RNA-Import Protein PNPase, Causes Hereditary Hearing Loss. Am J Hum Gen, 91(5):919-927.
- Rutherford MA, Pangršič T (2012) Molecular Anatomy and Physiology of Exocytosis in Sensory Hair Cells. Cell Calcium, 52(3-4):327-337.
- Rutherford MA, Chapochnikov NM, Moser T (2012) Spike Encoding of Neurotransmitter Release Timing by Spiral Ganglion Neurons of the Cochlea. J Neurosci, 32(14):4773-4789.
- Frank T, Rutherford MA, Strenzke N, Neef A, Pangršič T, Khimich D, Fetjova A, Gundelfinger ED, Liberman MC, Harke B, Bryan KE, Lee A, Egner A, Riedel D, Moser T (2010) Bassoon and the Synaptic Ribbon Organize Ca2+ Channels and Vesicles to Add Release Sites and Promote Refilling. Neuron, 68(4):724-738.
- Rutherford MA, Roberts WM (2009) Spikes and Membrane Potential Oscillations in Hair Cells Generate Periodic Afferent Activity in the Frog Sacculus. J Neurosci, 29(32):10025-10037.
- Roberts WM, Rutherford MA (2009) Linear and Nonlinear Processing in Hair Cells. J Exp Bio, 211(11):1775-1780.
- Rutherford MA, Roberts WM (2007) “Afferent Synaptic Mechanisms” In: The Senses: A Comprehensive Reference, Volume: Audition. Elsevier.
- Rutherford MA, Roberts WM (2006) Frequency Selectivity of Synaptic Exocytosis in Frog Saccular Hair Cells. Proc Natl Acad Sci USA, 103(8):2898-2903.
Contact Us
Mail address
Mark A. Rutherford
Dept. Otolaryngology, Campus Box 8115
Washington Univ. School of Medicine
660 So. Euclid Ave.
St. Louis, MO 63110
rutherfordmark@wustl.edu
Physical address
CID Building
Room 2215 (lab), 2278 (office)
4560 Clayton Ave.
St. Louis, MO 63110
lab phone: (314) 747-7152
office phone: (314) 747-7160
fax: (314) 362-1618