Our research employs state-of-the-art approaches, including in vivo two-photon imaging, confocal imaging, electrophysiology, inner ear drug delivery (intracochlear injection), and recently developed animal surgical procedures (cochleostomy and canalostomy). We monitor real-time calcium dynamics and ototoxic drugs in various types of cochlear cells in live animals under normal and pathological conditions. We utilize optogenetic tools to gain deeper insights into the functionality of cochlear cells. Our inner ear delivery method enhances the utility of in vivo cochlear imaging by precisely injecting the necessary sensors or drugs into the cochlea.
Sound coding and processing
In vivo multicellular imaging provides direct insight into peripheral sensory encoding at the systems level. Although in vivo multicellular responses at peripheral organs for sight, smell, taste, and touch have been investigated, similar functional assessment at the auditory periphery is hampered by its being mechanosensitive, fluid-filled, and encased in bone.
There is a knowledge gap in understanding how individual cell properties shape the cochlear mechanical responses as well as a gap in understanding how coordination between cells generates output either mechanically or synaptically. Our hearing-preserved cochleostomy creates an in vivo multicellular approach to the cochlea, allowing us to bridge the gap between cell and system function.
The first step for monitoring the functional multicellular activity using in vivo imaging is to find the most efficient sensors in the cochlea. We are exploring sensor technologies to monitor the cellular dynamics in the cochlea with the aim of targeting specific sensors for particular questions. Using the appropriate sensor, we will simultaneously monitor functional activity changes in multiple cochlear cells in response to the sound.
Our ultimate goal is to determine how cochlear cells receive and process complex sound input through in vivo real-time monitoring of the functional responses of both individuals and groups of cells.
Ototoxic drug tracking
Cisplatin is a highly effective chemotherapeutic drug; however, 40-80% of treated patients suffer from drug-induced hearing loss caused by irreversible hair cell degeneration.
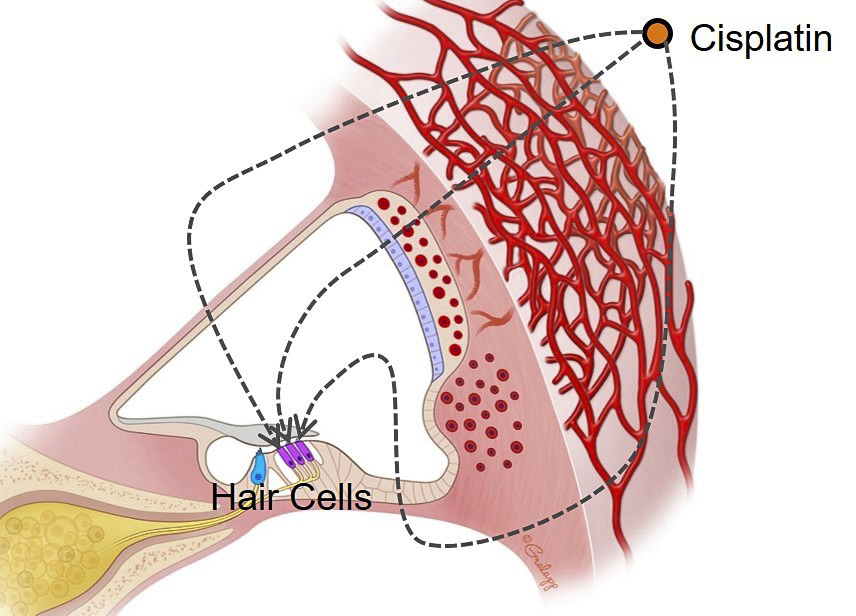
The mechanism by which cisplatin enters the cochlea and then targets hair cells is not fully understood. The possible entry routes of cisplatin into the cochlea or hair cells rely on data from fixed or neonatal tissue and are often based on in vitro assays, where cochlear compartments are compromised.
To overcome these limitations, we are utilizing the hearing-preserved in vivo multicellular cochlear imaing method, enabling us to track the drug in real time. Moreover, inner ear drug delivery through posterior semicircular canal provides another powerful tool to investigate which intracochlear fluid is involved in the pathway of drug transport.
Through real-time, in vivo monitoring of cisplatin with knock-out mutants/blockers of multiple transporters, we endeavor to identify the mechanism by which cisplatin is transported into the cochlea and hair cells. Furthermore, our goal is to pinpoint therapeutic targets that could mitigate cisplatin-induced hearing loss.
Ultimately, we aim to translate these studies into the clinical setting by testing the efficacy of potential protective therapies in patients receiving ototoxic drugs.