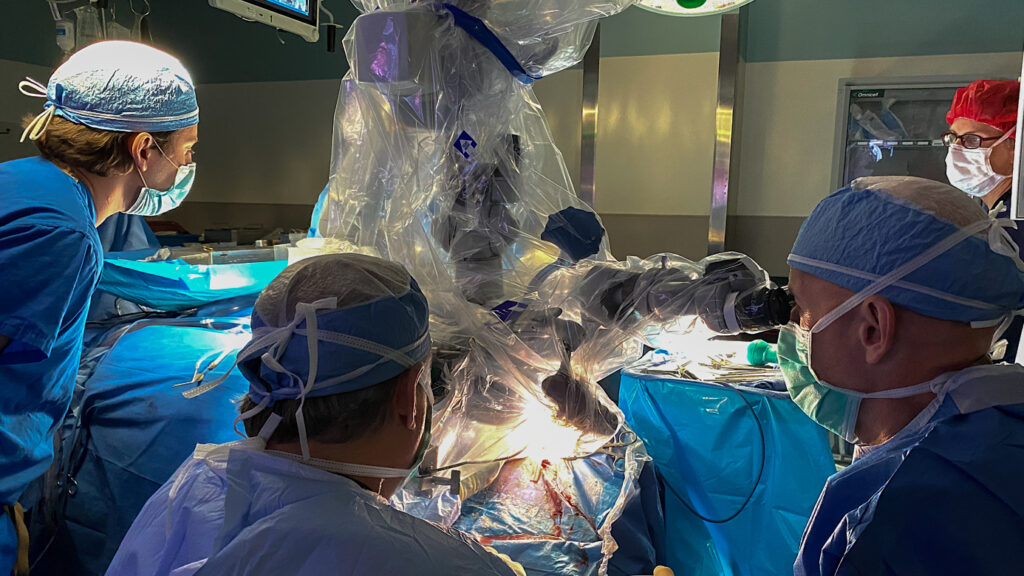
In a unique clinical trial, five acoustic neuroma patients have received cochlear implants at the time of their tumor removal. Early results are very encouraging.

Four of five patients have had their implants activated. Three of these have achieved sound awareness and more importantly demonstrated increased performance in word and sentence recognition tests compared to pre-surgery levels.
“Acoustic neuroma, also called a vestibular schwannoma, is a benign tumor on the auditory or vestibular nerves between the inner ear and brainstem,” explained Assistant Professor of Otolaryngology, Cameron Wick, MD, who is also principle investigator on the clinical trial. “Patients with this disorder are often forced to give up on their affected ear, so this trial provides real hope of restoring some of that function and improving patient quality of life.”
Many patients diagnosed with an acoustic neuroma lose function in the affected ear, leading to impaired hearing, tinnitus, and imbalance. If their tumor can be resected while maintaining the integrity of the auditory nerve, then a cochlear implant might restore that hearing
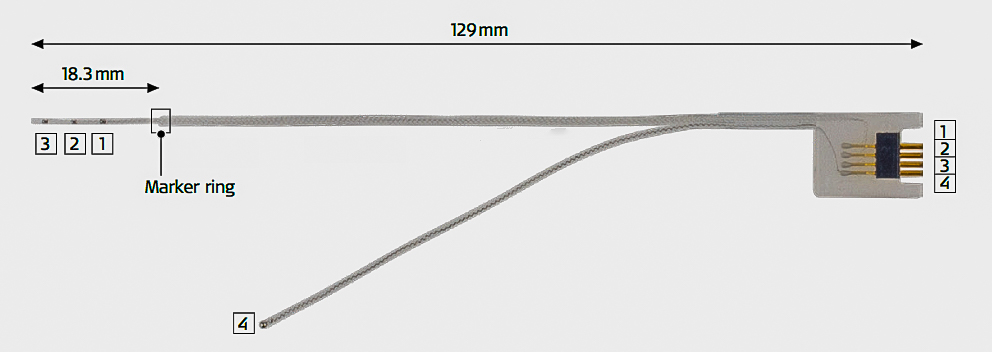
The ANTS is a novel system designed to facilitate auditory nerve monitoring. The device is comprised of three parts: a test electrode, connector cable, and stimulator box. During tumor resection the test electrode electrically stimulates the auditory nerve allowing surgeons to monitor the health of the nerve.
Outcomes measures will be evaluated in all patients at three, six, and 12 months post-activation. These tests will assess how well the cochlear implant is working, the cochlear implant’s impact on sound localization and hearing in background noise, and finally various aspects relevant to the patient’s quality of life: tinnitus, balance, hearing, and overall quality of life.
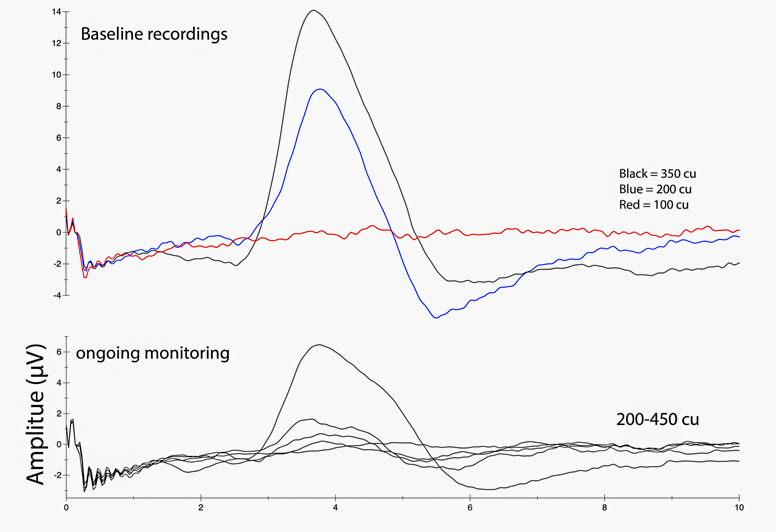
Wick is looking forward to enrolling more patients into the study. He is actively working on edits from the FDA regarding expansion of the trial to five additional patients, which he hopes to become available this spring. The team is currently vetting surgical candidates.
“Working with the FDA on a novel medical device is exciting, but also takes a team effort,” said Dr. Wick. “I am grateful to have such incredible support from my colleagues in otolaryngology and neurosurgery, including our research administrators.”
For additional information, contact Dr. Cameron Wick, or visit clinicaltrials.gov.