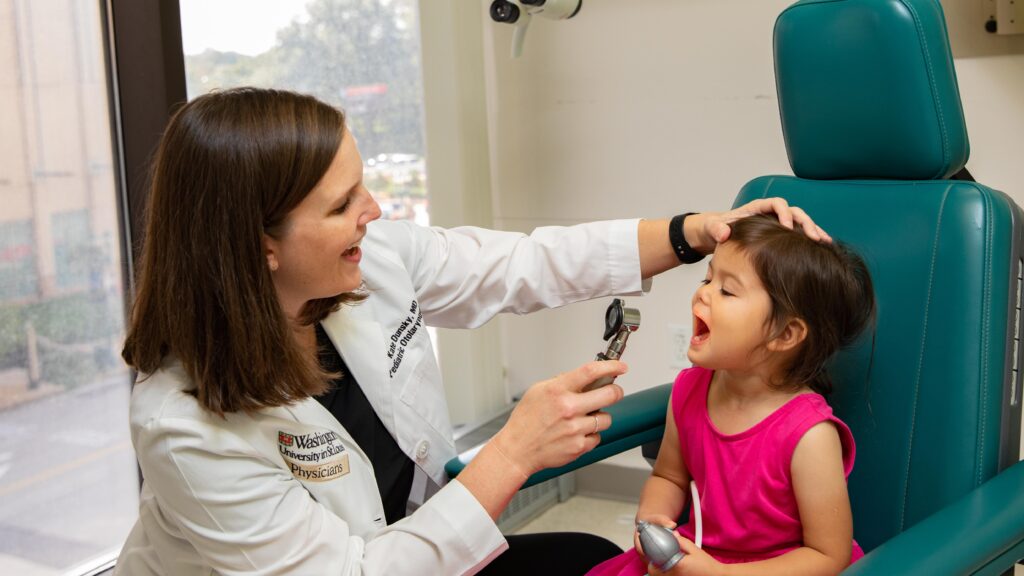A new clinical trial to improve post-tonsillectomy care at St. Louis Children’s Hospital started recruiting patients just a few weeks ago, but the study has been in design and preparation for over six months.
Co-Principal Investigator and Associate Professor in Otolaryngology David S. Leonard, MB, BCh, BAO, explained the need for a trial of this sort.
“Our motivation is really two-fold,” said Dr. Leonard. “First, we want to improve analgesia for post-tonsillectomy patients. Second, we hope that by improving these types of analgesic regimens, we can cut down on narcotic usage in children.”
According to Leonard, pediatric ENT specialists at Children’s Hospital have traditionally used alternating dosing so that patients are getting analgesia every three hours with the intent of providing the best overall pain control.

However, there is a theory that acetominophen and ibuprofen, when used together, have a synergistic effect.
“Synergism,” explained Leonard, “is when the combined effect of two treatments is greater than the sum of their individual contributions. This might improve on our current standard of care.”
A pilot group of 100 patients will be enrolled in the study. To date, 14 patients have been enrolled. St. Louis Children’s Hospital and the Children’s Specialty Care Center (CSCC) are the only recruitment sites, and will be the only locations for the pilot study. A larger study could be rolled out to other sites after the pilot study, if successful.
The effort is a collaboration between Washington University pediatric ENT specialists and Dr. Michael Montana from the Department of Anesthesiology at Washington University. Montana approaches this study from a broader post-operative care/analgesia mindset. The idea for the study came from a recent grand rounds presentation on narcotic reduction efforts by the Department of Anesthesiology. The study marks the first time that a clinical trial has been rolled out at an ambulatory care site like the CSCC. It is also the first study at Children’s Hospital to utilize an app called MyCap to collect data.